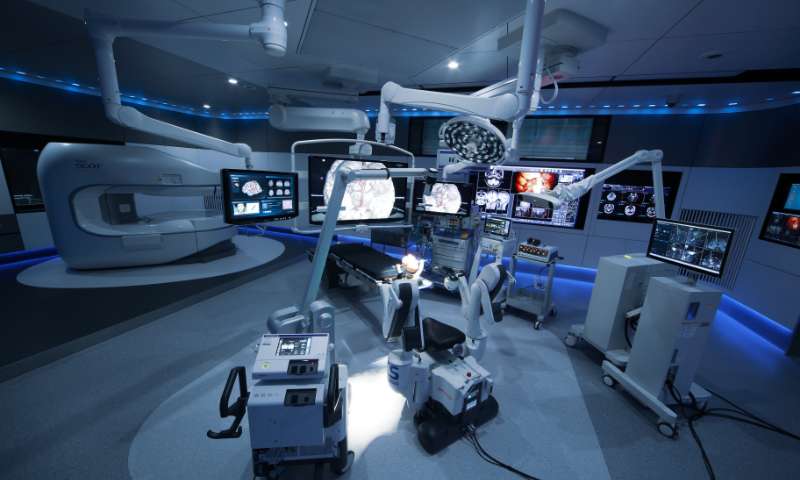
We are involved in product development without compromise in order to create attractive and appealing products in new markets.
Mizuho closely monitors market trends and forecasts in order to identify likely areas of future demand. Head Office teams work closely with engineers from the Chiba and Gosen factories to ensure that new products are closely aligned with the expectations and requirements of global markets. We also source quality products from overseas to further augment our extensive product range across multiple domains, while utilizing our production technology to manufacture OEM products.